Before the 20th century, medical care for the poor was primarily provided by private charities, often with a religious affiliation, or by hospitals and clinics funded by local governments. As hospitals evolved into the locus of medical care for the general population, charitable and publicly funded hospitals increasingly served more affluent patients as well. In response, those hospitals adopted financial strategies that effectively cross-subsidized the treatment of indigent patients with fees charged to patients who paid for their own care either directly or through insurance.
In the decades following World War II, Congress built on that basic design by enacting various programs and policies intended, in whole or part, to fund medical care for the indigent by subsidizing the institutions treating them. During the same period, Congress also enacted policies and programs that extended private and public health insurance coverage to more of the population.
The cumulative result is that, today, nearly all Americans have access to at least one source of health insurance, be it from public or private plans. Nevertheless, the federal government continues to steer more than $90 billion a year in direct and indirect indigent care subsidies to numerous medical institutions. This situation is largely due to vigorous interest group opposition to any attempt by Congress to “right size” indigent care funding as more of the uninsured gained coverage.
Compounding the situation is the current system’s reliance on funding mechanisms that are opaque, poorly targeted, lacking in accountability, and susceptible to manipulation.
The common source of both the political and operational problems with the current system is the fact that funding is distributed based on the characteristics of the recipient institutions rather than the characteristics of the patients being treated. Put another way, the current system operates as a “trickle down” approach to funding care for the indigent and uninsured.
This approach is a legacy of the historically provider-centric structure and administration of federal health care programs. Indeed, over the past 30 years, Congress created numerous Medicare and Medicaid hospital-payment adjustments based on institutional criteria. It is now nearly impossible for policymakers to accurately evaluate the effectiveness of those different funding streams, including the ones intended to offset indigent care costs.
Making the health care system more patient-centered requires reforms that give consumers more control over health care dollars and decisions, as well as reforms that incentivize providers to compete based on delivering better value for patients.REF For instance, the creation of Medicare Advantage gave seniors the ability to obtain coverage through the private plan of their choice, while the current implementation and expansion of health care price-transparency requirements is a predicate for enhancing provider competition.
Similarly, Congress should enact reforms that refocus existing indigent care funding on patients instead of institutions. Enacting patient-centered reforms that identify needy patients and target available funding to treating those patients will not only help those most in need but also will generate better data on the magnitude and distribution of uncompensated care costs. That, in turn, will establish greater transparency and accountability and give Congress a sounder basis for subsequently re-examining the total amount and allocation of funding.
Key Federal Indigent Care Programs and Policies
Today, the three largest sources of indigent care funding are the (1) Medicaid and (2) Medicare “disproportionate share hospital” (DSH) programs, and the (3) “340B” drug discount program. Those three programs are also the ones most characterized by poor targeting and lack of accountability, and thus in greatest need of reform.
1. Medicaid Disproportionate Share Hospital (DSH) Payments. When Congress enacted the Medicare and Medicaid programs in 1965, the legislation stipulated that hospitals were to be paid their “reasonable cost” for treating Medicare patients and that state Medicaid programs receiving matched federal funding were to pay hospitals on the same basis.
In response to rapidly increasing federal health care spending, Congress removed that requirement from Medicaid in 1981 and specified that state-set Medicaid payment rates were to “take into account the situation of hospitals which serve a disproportionate number of low-income patients with special needs.”REF The concern was that if states reduced the rates they paid hospitals for Medicaid patients, hospitals would have less revenue to offset the cost of providing charity care, with the hospitals that served more low-income patients being particularly disadvantaged. Subsequent changes in federal policy during the 1980s effectively encouraged states to increase DSH payments.
In the Omnibus Budget Reconciliation Act (OBRA) of 1986, Congress clarified that Medicaid’s hospital payment limitations did not apply to DSH payments. Subsequently, the 1987 OBRA required states to submit state plan amendments authorizing DSH payments. A 1985 federal regulation permitted states to use both public and private donations as sources of non-federal Medicaid financing, and a 1987 policy guidance indicated that taxes that were imposed on medical providers could also be used to finance Medicaid.REF As a result, in just the three years from 1990 to 1992, Medicaid DSH payments jumped 13-fold from $1.3 billion to $17.7 billion per year.REF
As Medicaid DSH spending rapidly increased, “federal policymakers grew concerned over both the level of DSH spending and the possibility that some states were misusing DSH funds by making large DSH payments to hospitals operated by state or local governments that were then transferred back to the state and used for other purposes.”REF
Congress responded in 1991 by imposing restrictions on states using provider taxes to fund their share of Medicaid. Additionally, the legislation imposed annual caps on the total amount of federal DSH funding along with limits on the share of that funding allocated to each state, using a formula based on state DSH spending in 1992.REF
When Congress enacted the Affordable Care Act (ACA) in 2010, it included provisions to lower the caps on total federal DSH funding over the six-year period of fiscal years 2014 through 2020.REF However, in response to hospital lobbying, Congress has so far postponed those funding reductions 13 times, and they have yet to take effect.REF
2. Medicare DSH and Uncompensated Care (UC) Payments. The origins and rationale for Medicare DSH payments are very similar to those for Medicaid DSH payments. The Social Security Amendments Act of 1983 changed in-patient hospital reimbursements under Medicare from “reasonable costs” to a system of prospective payments.REF Because of concerns that the new payment system would disadvantage hospitals that largely serve low-income patients, Congress included amendments in the Consolidated Omnibus Budget Reconciliation Act of 1985 establishing Medicare DSH payments.REF In 2010, provisions included in the ACA split Medicare DSH into two payments, labeling one “empirically justified DSH” and the other “uncompensated care,” with that change taking effect in 2014.REF
Functionally, Medicare DSH payments and UC payments take the form of (upward) adjustments applied to the standard rates paid to hospitals under Medicare’s Inpatient Prospective Payment System (IPPS).
These payments are based on formulas for determining both the hospitals that qualify to receive them and the size of the payment adjustment for each qualifying hospital.REF The payments are intended to defray costs incurred by hospitals for treating low-income uninsured patients. Because these payment adjustments are part of Medicare’s IPPS, they consist of adjustments to the payments received by hospitals for treating patients covered by traditional Medicare but not to payments that hospitals receive for treating patients covered by private Medicare Advantage plans.
3. The 340B Drug Pricing Program. Congress enacted the 340B Drug Pricing Program as part of the Veterans Health Care Act of 1992.REF The program was intended to provide pharmaceuticals at significant discounts to eligible clinics and hospitals (“covered entities”) that treat the poor and uninsured.
Under current law, for a drug to be covered by Medicaid and Medicare Part B, the manufacturer must participate in the Medicaid Drug Rebate Program and pay rebates to state Medicaid programs. The manufacturer must also provide even bigger discounts or rebates on the drug to 340B-covered entities and the Veterans Administration. Functionally, the 340B program constitutes an off-budget subsidy to qualifying clinics and hospitals.REF
Initially, participation in the 340B program was limited to clinics, health centers, and certain disease-specific or population-specific programs and facilities that receive federal grant funding, and to hospitals that qualify for Medicare DSH payment adjustments. In 2006, Congress expanded the list of institutions that are eligible to participate in the 340B program to include children’s hospitals, and further expanded the list of eligible institutions in 2010 to include those hospitals categorized in Medicare payment rules as free-standing cancer hospitals, critical access hospitals, rural referral centers, or sole community hospitals.REF
The scope of the 340B discount program has also expanded over time due to regulatory changes made by the Health Resources and Services Administration (HRSA), which administers the program, most notably with respect to so-called contract pharmacies. Because many health clinics did not have in-house pharmacies, in 1996 the HRSA issued guidance that permitted each eligible entity that did not have an in-house pharmacy to contract with a single outside pharmacy.REF Then in March 2010, the HRSA issued a revised notice that permitted 340B-covered entities to contract with multiple pharmacies.REF
Other Relevant Indigent Care Policies. The Medicaid and Medicare DSH programs and the 340B drug discount program also interact in important ways with two other federal policies.
One is the Emergency Medical Treatment and Active Labor Act (EMTALA). That law applies to any Medicare-participating hospital that has an emergency department (which is nearly every hospital) and requires those hospitals to provide emergency medical services to any individual in need, regardless of ability to pay or insurance status.REF Because EMTALA is effectively an unfunded federal mandate on hospitals to provide free, or deeply discounted, care to the uninsured, it gives hospitals another reason to seek compensatory government funding through DSH payments.REF
The other federal policy is federal funding for community health centers.REF As of 2021, 1,373 Federally Qualified Health Centers (FQHCs) operated 14,276 service delivery sites.REF Those clinics collectively received $5.2 billion in so-called Section 330 grants distributed by the HRSA, and another $4.2 billion in grants from other federal agencies, state and local governments, and private foundations.REF As noted, Congress originally created the 340B drug discount program primarily to help health clinics and hospitals that provide care to indigent patients. While clinics do not receive DSH funding, they are key stakeholders of the 340B program.
Time for Change
Although concern for uninsured Americans has long driven health policy debates, the reality is that nearly all Americans now have access to at least one private or public source of health coverage—including heavily subsidized coverage for those with low incomes. Currently, 156 million Americans (nearly half the population) are covered by employment-based plans, 16 million have individual market coverage, and about 10 million are covered through plans provided to federal workers and military families.REF Another 135 million Americans have public program coverage through Medicare, Medicaid, or the Children’s Health Insurance Program (CHIP).REF Thus, of an estimated total population of 333 million, it is likely that somewhere around 15 million to 20 million individuals are uninsured. Furthermore, the bulk of that residual uninsured population consists of individuals who did not enroll in coverage for which they are eligible.
A Congressional Budget Office (CBO) analysis of 2019 data found that two-thirds (67 percent) of the residual uninsured population were eligible for subsidized coverage through either an employment-based plan, the ACA exchanges, or the Medicaid and CHIP programs.REF The remaining one-third were ineligible for subsidized coverage because they were either individuals with incomes too high to qualify for ACA subsidies (9 percent of all uninsured);REF adults with incomes below the federal poverty level residing in states that have not adopted the Medicaid expansion (11 percent of all uninsured);REF or individuals “not lawfully present” in the U.S. (13 percent of all uninsured).
Given that two-thirds of the remaining uninsured are eligible for, but not enrolled in, private or public coverage, it is time for Congress to re-examine and reform the subsidies going to medical providers ostensibly for treating the indigent uninsured.
Yet, despite there now being significantly fewer low-income uninsured individuals without access to coverage, DSH funding levels have remained all but unchanged. To illustrate, Table 1 shows the current funding that hospitals and clinics receive for treating indigent uninsured patients. Those subsidies consist of $32.5 billion a year in federal government spending on Medicaid and Medicare DSH hospital payments and grants to health centers; $9.4 billion a year in state Medicaid DSH payments; and an estimated $52.3 billion a year in “off-budget” subsidies under the 340B drug discount program.
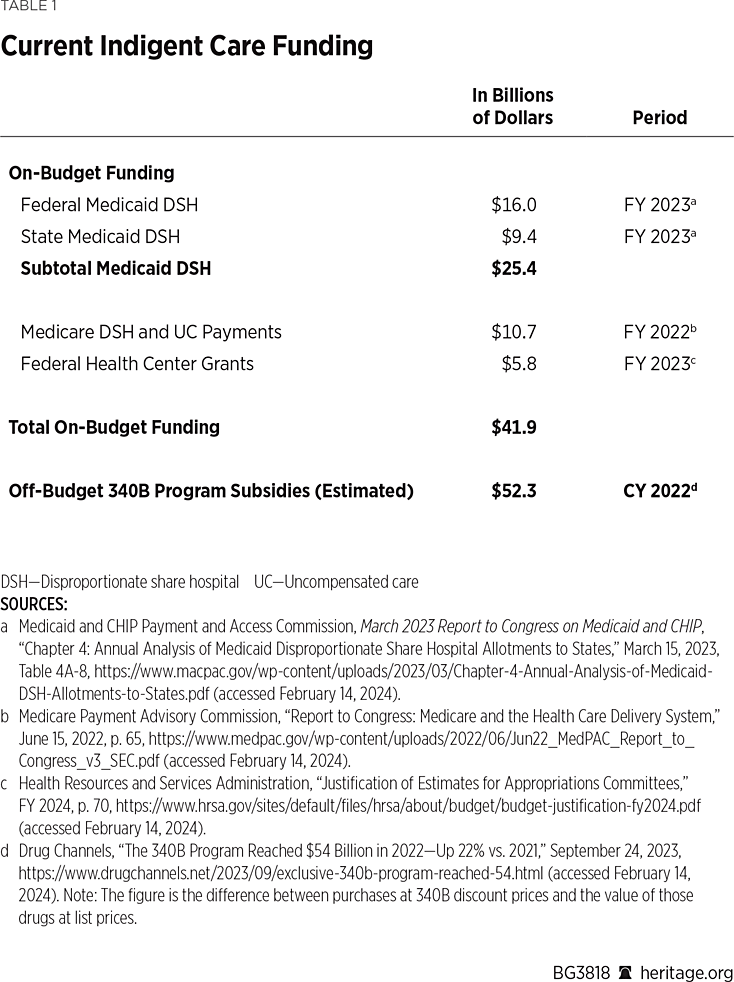
The problems with the current system all stem from the fact that funding is distributed based on the characteristics of the recipient institutions rather than the characteristics of the patients being treated. Put another way, the current system takes a “trickle down” approach to funding care for the indigent and uninsured. Meaning, funding is given to the institution under the assumption that it will be used to treat needy patients. As noted, even the ACA provisions to modestly reduce Medicaid DSH funding have been repeatedly delayed in response to hospital lobbying.
As a result, by funding institutions according to their characteristics and not the patient population, the Medicaid and Medicare DSH programs and the 340B program all suffer from opaqueness, poor targeting, lack of accountability, and susceptibility to manipulation. That is true despite current reporting requirements, as most of the information reported is for proxy metrics. None of the current reporting results in data suitable for identifying the actual treatment costs of a specific patient or an uninsured patient’s eligibility for subsidized health insurance coverage.
Problems with the Medicaid DSH Program
Federal Medicaid DSH funding is still allocated among the states largely based on accidental historical patterns. Similarly, state distribution of Medicaid DSH funds to hospitals relies on proxy measures that poorly correlate with the actual amount of uncompensated care provided by each hospital.
Despite Congress adjusting the allocation formula over the years, wide disparities still exist. In its March 2019 report to Congress, the Medicaid and CHIP Payment and Access Commission (MACPAC) stated, “As in our past reports, we find little meaningful relationship between current DSH allotments and the factors that Congress asked MACPAC to consider.”REF The report went on to note that the state DSH allotments for fiscal year 2019 ranged “from less than $100 per uninsured individual in five states to more than $1,000 per uninsured individual in nine states,” and that when measured another way—according to the amounts of charity care reported by hospitals in their 2016 Medicare cost reports—the state allotments ranged “from less than 10 percent in six states to more than 80 percent in six states.”REF
At the state level, the distribution of Medicaid DSH funds to hospitals is also characterized by wide variations. The current situation is the product of a combination of federal rules and state funding decisions.
The federal Medicaid statute specifies that:
- States must provide DSH payments to any hospital that meets the federal criteria for being “deemed to be a disproportionate share hospital.” To qualify for that designation, the hospital must either have a “Medicaid inpatient utilization rate,” that is “at least one standard deviation above the mean” for all hospitals in the state that receive Medicaid payments, or have a “low-income utilization rate” of 25 percent or greater, which is calculated based on costs, not patient volume.REF
- A state may provide DSH payments to any hospital with a Medicaid inpatient utilization rate of at least 1 percent.REF A hospital’s Medicaid inpatient utilization rate is the percentage of total inpatient days attributable to Medicaid patients.REF
- A state’s DSH payments to a hospital cannot exceed the total amount of uncompensated care provided by the hospital to Medicaid and uninsured patients—calculated as the hospital’s cost for treating Medicaid and uninsured patients, minus the total amount of other Medicaid reimbursements and supplemental payments plus all payments from uninsured patients.REF
Within those federal guidelines, states have relatively broad discretion in allocating DSH funds to hospitals. Nationally, 42 percent of all hospitals receive some DSH funding, but there is considerable variation among states. Some states provide Medicaid DSH payments to less than 10 percent of their hospitals, while a few other states provide DSH funding to more than 80 percent of their hospitals.REF
While Medicaid DSH payments are intended to fund care for indigent uninsured patients, a hospital may also receive other types of Medicaid supplemental payments. States can manipulate how funds flow to specific hospitals by working within the federal rules governing different types of Medicaid supplemental payments.
For instance, under federal Medicaid rules hospital “uncompensated care” includes not only the cost of treating indigent uninsured patients but also any “Medicaid shortfall”—calculated as the difference between a hospital’s costs for treating Medicaid patients and the Medicaid “base rate” reimbursements it received for those patients. Therefore, a state that pays its hospitals low Medicaid base rates could use DSH funding to cover part, or even all, of the “Medicaid shortfall” of a particular hospital—even if the hospital did not treat any indigent uninsured patients.
Similarly, federal rules say that state Medicaid programs cannot reimburse hospitals for treating Medicaid patients more than what Medicare would have paid for treating the same patients. However, states are allowed to calculate an upper payment limit (UPL) on an aggregate basis by hospital ownership class (private, state government, local government) and provide supplemental payments to only some hospitals within the applicable class. As MACPAC notes, this means that “UPL payments to individual hospitals can exceed the hospitals’ costs as long as total payments for each class of providers are below the UPL.”REF
By setting its Medicaid base payment rates at low levels and then manipulating the distribution of DSH and UPL supplemental payments to hospitals, a state can steer more Medicaid funding to certain hospitals. Consequently, some states deliberately direct the bulk of their DSH and other supplemental payments to public (usually local government-owned) hospitals, thus forcing private (usually nonprofit) hospitals to cost-shift their losses from treating Medicaid and uninsured patients to private payers.
Problems with Medicare’s DSH and UC Payments
Medicare DSH payment adjustments were originally established in 1985. A provision included in the ACA split Medicare DSH funding, starting in 2014, into two payments calculated using different formulas. One-quarter of the amount that would have been paid to hospitals based on the previous DSH formula is now paid based on a calculation of “empirically justified DSH,” while the other three-quarters of the funding is now distributed to hospitals as “uncompensated care” payments. Those uncompensated care payments are also annually adjusted to reflect the percentage change in the non-elderly national uninsured rate since 2013 and each eligible hospital’s share of total uncompensated care costs. Congress’s objective in revising the payment design was to adjust uncompensated care payments downward as the uninsured rate fell and also to adjust for increases or decreases over time in each hospital’s uncompensated care costs relative to all other eligible hospitals.
A 2007 analysis by the Medicare Payment Advisory Commission (MedPAC) found Medicare DSH payments to be poorly targeted.REF Despite Congress revising the payment methodology, subsequent MedPAC analyses still found flaws with the current design, which largely result from continued reliance on proxy measures. In particular, MedPAC notes that the payment formulas still disadvantage hospitals that treat a larger share of indigent uninsured patients. Also, the formulas still adjust payments to hospitals for inpatient care under Medicare Part A, but not payments for outpatient care under Medicare Part B.
Consequently, the current formulas do not account for the technology-driven shift of more procedures and services to outpatient settings or the cost to hospitals of providing outpatient treatments to indigent patients. Furthermore, while 82 percent of urban hospitals and 92 percent of rural hospitals are now eligible for these payments, the remaining hospitals are unable to claim these offsets against their uncompensated care costs.REF
Problems with the 340B Drug Pricing Program
Like hospital DSH payments, the 340B discount program was intended to subsidize the cost of providing medical care to indigent uninsured patients. Both DSH and 340B are poorly targeted because both are structured to subsidize institutions, as opposed to directly assisting patients. The main functional difference is that DSH payments are “on budget” expenditures by federal and state governments, while 340B subsidies are “off budget” funding extracted from drugmakers and public and private insurance plans.
The principal problem with the 340B program is that covered entities can claim discounts not only for drugs prescribed for their uninsured patients but also for the drugs they prescribe for their insured patients.
While 340B hospitals and clinics do serve many indigent and uninsured patients, most of their patients have public or private insurance that covers prescription drugs. For instance, of the 30 million patients served by FQHCs that receive federal grant funding, only 20 percent are uninsured, while about half are Medicaid enrollees, 10 percent are covered by Medicare, and 20 percent have private insurance.REF
Under the program’s current structure, 340B entities can charge an insured patient’s health plan the full cost of the drug, while obtaining the drug at its steeply discounted 340B price. Thus, covered entities can arbitrage the disparities between their drug acquisition costs and insurer reimbursements to pocket the difference. Furthermore, 340B entities can share those proceeds with pharmacies and pharmacy benefit managers (PBMs) in exchange for the pharmacies and PBMs facilitating the transactions.
Congress expected that covered entities would use the 340B program to generate operating revenues, but to limit the program’s scope, Congress also included provisions specifying which institutions could participate. It prohibited covered entities from reselling or transferring discounted drugs to an individual “who is not a patient of the entity” and specified that for Medicaid enrollees treated by covered entities, either the 340B discount or the Medicaid rebate could be applied, but not both.REF Because the initial focus of the 340B program was primarily on clinics that receive grant funding from the HRSA, Congress tasked that agency with administering the program.
Since 2010, the program has expanded significantly due to a combination of Congress amending the participation criteria to include more hospitals and the HRSA issuing regulations that define who is an eligible patient in broad terms and that permit covered entities to contract with more pharmacies and pharmacy benefit managers.
Table 2 shows how the 340B program has exponentially expanded over the past 12 years.
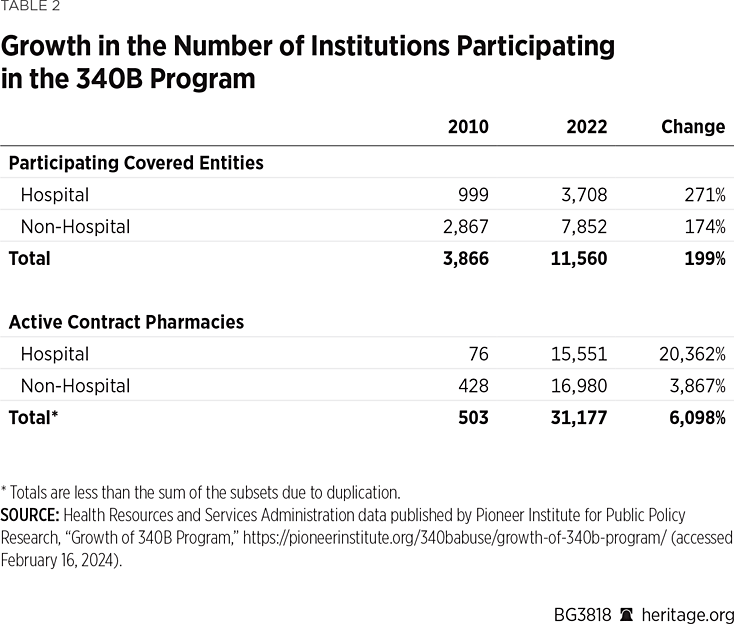
Of course, the expansion of the program resulted in more drugs being acquired at 340B prices and larger subsidies flowing to 340B entities and their contract partners. Indeed, one analysis observed that “[t]his ‘buy low, sell low’ program has evolved into a ‘buy low, sell high’ program that enables eligible hospitals to generate profits by providing these drugs to well-insured patients.”REF
Analyses of 340B drug purchase data find that, in 2015, drugs purchased by 340B entities had a list price value of $32.6 billion but that their acquisition cost at 340B prices was $12.2 billion. By 2022, those purchases had grown to a list price value of $106 billion and 340B acquisition costs of $53.7 billion. Thus, the difference between list prices and 340B prices indicates that the 340B program generated subsidies to covered entities (and their pharmacy and PBM partners) of around $20.4 billion in 2015, rising to $52.3 billion in 2022.REF The analysis also found that 87 percent of 340B purchases were made by hospitals, while only 13 percent were made by clinics—which were the initial focus of the program.
The 340B program’s enormous growth since 2010 has also exacerbated the program’s other flaws, namely:
- The increasing capture of subsidy dollars by large corporate entities, most notably chain drug stores, pharmacy benefit managers, and major hospital systems.REF One factor is the use of contracts structured to pay the pharmacy a share of the revenue derived by the covered entity, rather than just fixed fees.REF
- Inadequate federal oversight to prevent duplicate discounts and the diversion of drugs to ineligible patients.REF
- Insured patients paying higher copays because cost-sharing provisions of their coverage are linked to list prices rather than the discounted prices actually paid for the drugs.REF
- Incentives for hospitals to designate newly built or acquired locations in wealthy areas as “child sites” to extend the 340B discounts to them as well. Many of those locations are for medical specialties that tend to prescribe newer and more expensive drugs, such as oncology.REF
- Perverse incentives for drug companies to compensate for providing substantial rebates and discounts to more patients by setting higher initial launch prices for new drugs. Those incentives have been exacerbated in recent years by Congress enacting other provisions requiring drugmakers to pay additional Medicaid and Medicare rebates when they increase prices for drugs already on the market by more than the general inflation rate.REF
- Growing concern that hospitals are not using the savings they receive through the 340B program to offset indigent care costs, and that the program may have created incentives to shift care from physician offices to more expensive hospital out-patient settings.REF Indeed, one analyst notes, “Unlike non-hospital covered entities, 340B DSH hospitals are not required to use 340B savings to serve vulnerable populations, nor are they required to report how 340B revenues are used.”REF
Guiding Principles for Reforming Indigent Care
The principal goal of reform should be to target funding to meet actual needs. To that end, the key outcomes for a reformed system are that it more precisely identifies the patients who need help and focuses the available funding on treating those patients.
Finally, reforming indigent care subsidies is an important first step toward creating greater price transparency and program accountability. That is particularly important with respect to hospital financing, which is notoriously opaque and riddled with explicit and implicit cross subsidies. Congress has compounded that situation by creating, over the past 30 years, numerous Medicare and Medicaid hospital payment adjustments for different purposes—all based on institutional criteria. As a result, it is now nearly impossible for policymakers to accurately evaluate the effectiveness of those different funding streams, including the ones intended to offset indigent care costs. Reforms would produce the data needed to make better evaluations of actual needs and appropriate funding levels.
How Congress Should Reform Medicaid and Medicare Hospital Payments for Indigent Care. Today, the allocation of federal Medicaid DSH funding among the states is largely based on accidental historical patterns, while the distribution of Medicaid DSH funds to hospitals is mainly based on proxy measures that poorly correlate with need. Similarly, Medicare’s DSH and UC payment adjustments are based on the same fundamental design flaw of using proxy measures that poorly correlate with need.
Because Congress expected hospital uncompensated care costs to decline as the ACA extended subsidized health insurance coverage to more low-income individuals, the legislation also included provisions to gradually reduce federal funding for Medicaid and Medicare DSH. Ten years later, millions of previously uninsured individuals now have coverage, and fully two-thirds of the remaining uninsured are eligible for subsidized coverage. However, persistent lobbying by hospitals has successfully prevented the scheduled Medicaid DSH cuts from ever taking effect.
Clearly, Congress’s strategy of imposing DSH funding cuts to drive reform of hospital spending has failed. Congress should now pursue the reverse approach of first reforming DSH and later revising federal funding to track declines in uncompensated care costs.
Congress should start by restructuring existing federal Medicaid and Medicare DSH funding into a new Medicaid uncompensated care pool (UCP) program that subsidizes indigent care on a more transparent, equitable, and accountable basis.REF
While state participation in the consolidated and reformed Medicaid UCP program would continue to be voluntary, federal funding should be conditioned on the state establishing a state-wide UCP that provides claims-based reimbursement to hospitals for uncompensated care. In other words, a hospital would have to submit to the state pool a claim that identifies the treated individual and the services provided—just as it does for patients covered by private or public insurance plans. Furthermore, the state pools would be required to accept claims from all hospitals within their state that are subject to EMTALA.
This design would ensure equal treatment for all hospitals—which is not currently the case in most states. It would also provide policymakers with better information on the patients treated and the services provided.
Within those basic parameters, each state would select a fee schedule to apply to pool claims and determine the process and timing for adjudicating pool claims.REF For any period for which the available funding exceeds the total amount of pending claims, the pool would pay all claims according to the established fee schedule. For any period for which the total amount of pending claims exceeds the available funding, the pool would pay all claims on a pro rata basis.
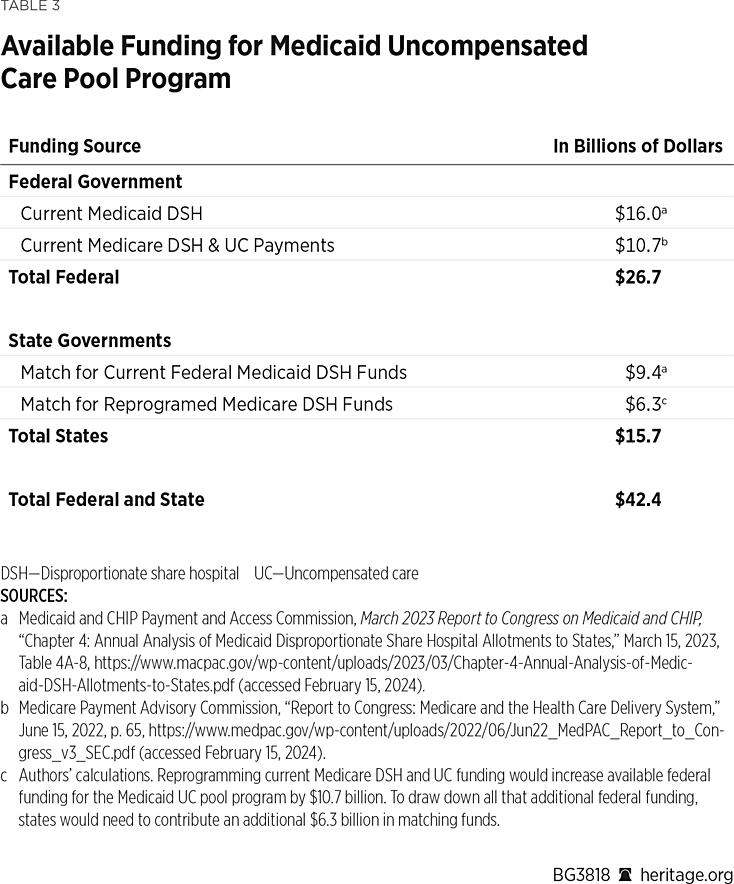
Federal funding for the new UCP program should initially be set at the same amount as current Medicaid DSH ($16 billion in 2023) plus what Medicare now spends on DSH and UC hospital payment adjustments ($10.7 billion in 2022).
To maintain a shared federal and state responsibility, the new UCP program would operate under Medicaid and states would have to contribute funding based on each state’s standard Federal Medical Assistance Percentage (FMAP) rate—just as they currently do for Medicaid DSH.
As Table 3 shows, reprograming current Medicaid and Medicare DSH spending would initially provide the new Medicaid UCP program with more than $42 billion a year in available funding. That should be enough to cover all hospital claims for treating indigent uninsured patients.
The new Medicaid UCP program will inherently generate better data on the magnitude and distribution of the costs incurred for treating indigent uninsured patients. Consequently, once the program has been operating for several years, Congress will have a more accurate picture of both the national cost of uncompensated care and each state’s share. That will enable Congress to more appropriately revise both the overall level of federal funding and the apportionment of that funding among the states.REF
How Congress Should Reform the 340B Drug Discount Program. As noted, the 340B program currently generates more than $50 billion in “off budget” subsidies to hospitals, clinics, contract pharmacies, and PBMs. Obviously, those vested interests have an enormous financial stake in maintaining the status quo, and thus pose a significant obstacle to reforming the program.
The best course for Congress is to enact a series of reforms designed to gradually reduce the level of unwarranted institutional subsidies while targeting discounted drugs more accurately to needy patients.
Congress should start by limiting 340B entities to designating only one contract pharmacy (but permit the HRSA to waive that restriction for entities without in-house pharmacies) and require the HRSA to contract with a neutral and independent 340B claims data clearinghouse to facilitate verification of 340B claim eligibility.
Limiting the number of contract pharmacies will reduce the opportunities for chain drug stores and PBMs to abuse the program by skimming off revenue for themselves. At the same time, allowing waivers from the “one contract pharmacy” rule for entities without in-house pharmacies will avoid disrupting non-abusive arrangements.REF Creating a 340B claims data clearinghouse would provide a better mechanism for identifying and correcting instances of improper duplicate discounts. The creation of a 340B claims data clearinghouse is a reform that already has support from key stakeholders.REF
The next step should be for Congress to require the HRSA to contract with private vendors to adjudicate claims for prescriptions written by 340B-covered entities. Medicare has long used a similar approach of contracting with private vendors (called Medicare Administrative Contractors) to process physician and hospital claims for enrollees covered under traditional Medicare.
Each contract would apply to a specified area (such as a state or region) and the vendors would be responsible for determining a patient’s applicable drug coverage, any split billing (such as when a patient presents a manufacturer coupon or a discount card), and any coordination of benefits (if a patient is covered by more than one health plan).
If a patient has public or private drug coverage, the vendor would obtain reimbursement from the patient’s plan and pass it on to the dispensing pharmacy. If the patient is uninsured, the dispensing pharmacy would pay the 340B price for the drug, and the entity that originated the prescription would determine the patient’s co-payment amount (if any) and would reimburse the pharmacy for the difference between the patient’s co-pay and the pharmacy’s acquisition cost plus a dispensing fee.
Over time, the functional effect of the adjudication system would be to limit 340B discounts to the drugs consumed by uninsured patients—which was Congress’s original intent for the program.
As the final step, Congress should integrate the reformed 340B program with the proposed reforms to DSH payments. Specifically, Congress should include in the updated Medicaid UCP program a provision permitting state UCPs to also pay claims from 340B entities (including those that are not hospitals) for their unreimbursed expenses associated with providing discounted drugs to low-income uninsured patients. Such claims would be for the amount of subsidy provided by the hospital or clinic to the patient, calculated as the difference between the drug’s 340B acquisition and dispensing costs and the patient’s direct payment for the drug.
Integrating the two sets of reforms in this fashion would result in a more coherent design for directly subsidizing medical care provided to indigent patients. Once that is achieved, there would no longer be any need for Congress to limit pharmacy contracting by 340B entities or to restrict participation in the state UCPs to only hospitals. Under a reformed and integrated system, both hospitals and clinics would have a simpler, clearer, and more accurate way to obtain reimbursement for treating indigent patients.
Conclusion
Today, nearly all Americans have access to one or more sources of health insurance, including heavily subsidized coverage for those with low incomes. Yet, federal programs and policies continue to provide hospitals and clinics with more than $90 billion a year in direct and indirect indigent care subsidies. That level of funding is likely much greater than the need, given that America now has functionally near-universal health insurance coverage.
Furthermore, the principal sources of that funding are the Medicaid and Medicare DSH programs and the 340B program—each of which is characterized by opaqueness, poor targeting, lack of accountability, and susceptibility to manipulation.
Those problems all stem from the fact that funding is currently distributed based on the characteristics of the recipient institutions rather than the characteristics of the patients being served. Put another way, the current system operates as a “trickle down” approach to funding care for the indigent and uninsured.
It is time for Congress to reform those programs and policies to target existing funding to meet actual needs. To that end, the key outcomes for a reformed system should be that it specifically identifies the patients who need help and focuses available funding on treating them.
Reforming indigent care subsidies is also an important step toward creating greater price transparency and program accountability. That is particularly important with respect to hospital financing, which is notoriously opaque and riddled with explicit and implicit cross subsidies.
In sum, making the health care system more patient-centered requires reforms that give consumers more control over health care dollars and decisions, as well as reforms that incentivize providers to compete based on delivering better value to patients. Reforming current funding for indigent care to focus on patients rather than institutions will not only do a better job of directing assistance to those most in need but will also compliment broader efforts to make government spending on medical care more transparent and accountable.
Edmund F. Haislmaier is Senior Research Fellow in the Center for Health and Welfare Policy at The Heritage Foundation. Miles Pollard is a Policy Analyst for Economic Policy in the Center for Energy, Climate, and Environment at The Heritage Foundation.



