No modern American President has come close to matching Franklin Delano Roosevelt in the scope of actions taken during the first 100 days in the Oval Office. Just 48 hours after his inauguration, he suspended all banking transactions, and two days later began drafting legislation to seize regulatory control of the entire financial system. He also launched a public works spending spree, introduced agricultural subsidies, and initiated government production of energy, all in his first three months. From this burst of executive power was coined the term “the First 100 Days” as a measure of leadership and political strength.
There is unlikely another period in an executive’s term when the opportunity for reform is as great and the political capital as ample—particularly if the margin of election victory was significant.
Not that a new President enjoys free rein. The U.S. Constitution, if honored, limits a President’s power to act unilaterally. For example, the White House alone cannot rescind regulations mandated by statute, although a President appoints agency heads who exercise latitude in rulemaking priorities.
Presidents may also dictate some agency actions through executive orders and guidance documents. With the Office of Management and Budget (OMB) ensconced within the Executive Office of the President, there are a variety of opportunities for influence.
President Donald Trump will need all of the means available to him to countermand the injurious policies inflicted on the nation by the Obama Administration (with help from Congress) during the past eight years.
Among the worst is the unparalleled growth in the number and cost of regulations.
Scope of the Problem
Federal regulators have issued more than 22,700 regulations since the start of the Obama Administration in 2009, which increased regulatory costs by more than $120 billion each and every year.[REF] The actual costs are far greater, both because the impacts have not been fully quantified for a significant number of rules, and because many of the worst effects—the loss of freedom and opportunity—are incalculable.
Regulation acts as a stealth tax on Americans and the U.S. economy. The weight of this tax is crushing, with independent estimates of total regulatory costs exceeding $2 trillion annually—more than is collected in income taxes each year.
As the number of regulations has grown, so, too, has spending on government bureaucracy. Based on fiscal year 2017 budget figures, administering red tape will cost taxpayers nearly $70 billion, an increase of 97 percent since 2000. A big part of the increase is the growing legions of regulators—who now number an all-time high of 279,000.[REF]
Instruments of Reform
For purposes of steering regulatory policy, the President’s authority to appoint the heads of executive branch agencies (under the Appointments Clause of the Constitution[REF]) is among the most effective. The President is not obliged to seek advice from the Senate about selecting a nominee and, therefore, is free to choose someone who embraces his policy prescriptions.
Perhaps even more potent is the President’s power to dismiss agency secretaries, administrators, and directors, should they deviate from the White House agenda.[REF] Most of the President’s appointees serve at his pleasure.
As with most grants of power, however, the Constitution balances the President’s appointments authority by requiring Senate confirmation of the nominees and congressional control of regulation via statute.
The heads of independent agencies do not serve at the pleasure of the President. In most instances, commissioners serve terms fixed in statute and may only be dismissed “for cause.” However, staggered terms ensure that even a one-term President has an opportunity to appoint at least one commissioner, and thus secure a majority in most instances. Moreover, the President, in most cases, has authority to designate a commission chair to oversee the staff and steer the agenda.
The President also wields budgetary influence over regulatory agencies. Individual agencies submit budget requests to the OMB, which formulates a proposed budget in accordance with the Administration’s priorities. The President’s budget submitted to Congress will reflect, in part, the extent to which he or she approves or disapproves of various agency actions—regulatory and otherwise. Ultimately, however, Congress determines the level of appropriations.
Another tool is control of litigation through the Department of Justice. Generally speaking, cabinet agencies rely on the Justice Department to litigate on their behalf, which means that the President (through his appointees) can influence how cases are prioritized and resources are deployed. The Trump Administration would do well to review all pending litigation and designate cases for settlement, including challenges to Obama’s untenable Clean Power Plan;[REF] his radical transgender bathroom directive;[REF] and the Environmental Protection Agency’s (EPA’s) egregious “waters of the U.S.” rule.[REF]
Executive orders (EOs) represent a direct means by which the President establishes his or her policies, although the President cannot override statutory directives to agencies unless the law expressly grants that power. Barack Obama issued a total of 277 EOs, the number of which actually lags behind George W. Bush’s 291 and Bill Clinton’s 364. However, the number alone does not convey the scope of a President’s unilateral action (which also includes presidential memoranda). Although the number of Obama EOs broke no records, he was particularly aggressive in using executive orders to thwart the will of Congress—on immigration, labor, and the environment, in particular.
President Trump has lost no time in issuing his own EOs, and he is free to rescind any of his predecessors’ orders—many of which deserve to be hastily dispatched. (See Appendix 1 for recommended EO rescissions.)
Time Out
The day that President Trump took office, his Administration inherited 1,985 regulations in the rulemaking pipeline—966 in the proposed stage, and 1,019 in the final stage.[REF] Like his predecessors, the President’s first actions included a regulatory freeze in the form of a memorandum to executive departments and agencies (with an overly broad exception for “emergency or other urgent circumstances relating to health, safety, financial, or national security matters, or otherwise”).[REF]
The memo directs agency heads to:
- Refrain from sending regulations[REF] to the Office of the Federal Register until a department or agency head designated by the President reviews and approves it. (Publication in the Federal Register is required to finalize a rule.)
- Withdraw regulations that had been sent to the Office of the Federal Register but have not yet been published.
- Postpone, for 60 days, regulations that have been published in the Federal Register but have not yet taken effect, for the purpose of reviewing questions of fact, law, and policy (as permitted by law).
For regulations that conflict with the new Administration’s policies—which, it is to be hoped, will constitute a very large number—agencies may propose either to further delay the effective date or to rewrite or repeal a rule. However, this requires following the rulemaking process and providing justification subject to public notice and comment. Though time-consuming, the effort is justified to overturn particularly egregious regulations, such as the EPA’s Greenhouse Gas Endangerment Finding and the Renewable Fuel Standard, and the unpalatable school lunch standards imposed by the Department of Agriculture.
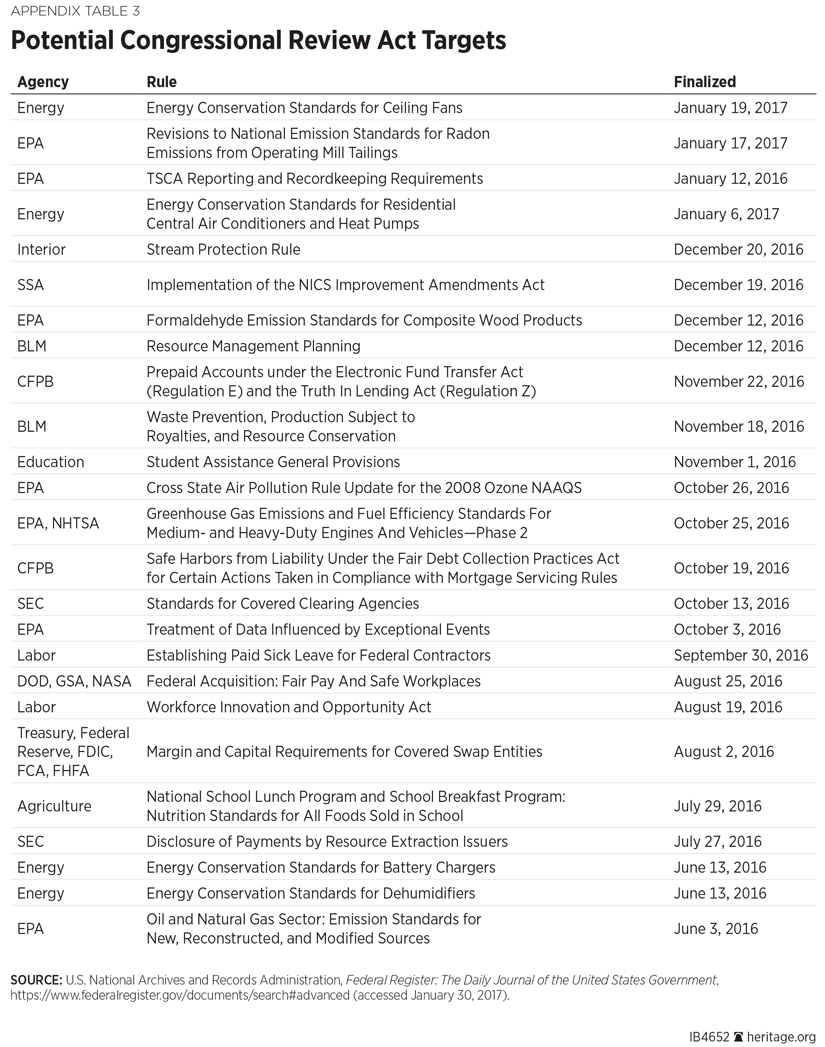
The Congressional Review Act (CRA) provides a legislative means of repealing regulations that have been finalized within the past 60 days (with exceptions[REF]). Doing so requires a resolution of disapproval passed by Congress, and the President’s signature.
For each CRA-targeted rule, a joint resolution of disapproval must be introduced within 60 legislative days of finalization of the rule. The resolution may be considered by the House and Senate for 60 legislative days.
Senate rules allow for expedited consideration of the proposed resolution. If the committee of referral does not report the resolution within 20 legislative days of receipt, the resolution may be discharged by a written consideration of 30 senators. Once discharged, the measure is placed on the Senate calendar. Thereafter, it is in order at any time for a motion to proceed to consideration. All points of order against the joint resolution (and against consideration of the measure) are waived, and the motion is not subject to debate, amendment, postponement, or to a motion to proceed to other business.
Only a simple majority threshold is required for passage of the resolution (218 votes in the House; 51 votes in the Senate). Approval of a resolution prohibits an agency from issuing a substantially similar regulation unless authorized by Congress, and the resolution is not subject to judicial review. (See Appendix 3 for recommendations.)
The Power of Regulatory Review
The ultimate White House influence on rulemaking may well be the regulatory review process administered by the OMB. Specifically, the OMB’s Office of Information and Regulatory Affairs (OIRA) is responsible for reviewing proposed and final regulations, managing agency requests for information collection, and overseeing data quality government-wide.
The power of regulatory review is evidenced by the attention paid to it by each new Administration. Every President over the past four decades has customized regulatory review procedures. (See Appendix 3.) And no wonder. OIRA determines whether agencies have complied with rulemaking requirements, including the integrity of risk assessments and cost-benefit analyses, and controls if and when a regulation is finalized. That is real power in an era of regulatory overload.
The stringency of OIRA’s regulatory review is largely the prerogative of the President, and is established by executive order. In its current incarnation, OIRA’s regulatory review is overwhelmed by the volume of rulemaking. With a current staff of about 50, it is reviewing the work of agencies that have a combined total of 279,000 personnel, a ratio of more than 5,600 to 1.
The Trump Administration should replace the existing regime with stricter standards for review, a broader scope of review, and greater transparency in the review process. Among other elements, the new order should:
- Require independent agencies to comply with all rulemaking requirements under the Paperwork Reduction Act, the Unfunded Mandates Reform Act, the Data Quality Act, and all other rules that apply to executive branch agencies.
- Require agencies to submit all regulations, not just significant regulations, to OIRA.
- Require agencies to conduct a regulatory impact assessment for guidance documents, policy memos, and rule interpretations.
- Require agencies to base decisions on factual data, and to fully disclose any such data and the basis of a proposed decision in a manner that allows critical review by the public.
- Disallow rulemaking that assesses risk based on a “No Safe Threshold” linear regression analysis, which assumes that any chemical posing a health threat at a high exposure will also pose a health threat at any exposure level, no matter how low.
- Reject any rulemaking for which the benefits exceed the cost only by reliance on “co-benefits.” (The term refers to ancillary outcomes that are quantified to make it appear that the rule’s benefits exceed the costs when the actual focus of the regulation does not justify the regulatory cost.)
Legislation
The Trump Administration should also promote congressional consideration and passage of regulatory reforms that would:
- Amend the Congressional Review Act to allow a single resolution of disapproval to address multiple regulations.
- Require congressional approval of new major regulations.
- Make retrospective review more effective by requiring sunset dates for all major regulations. Rules should expire automatically if not explicitly reaffirmed by the relevant agency through the formal rulemaking process. As with any such regulatory decision, this reaffirmation would be subject to review by the courts.
- Codify regulatory impact analysis requirements.
- Secure congressional modification of the scope of judicial review of agency actions to authorize courts reviewing agency actions to decide de novo (without giving deference to the agency’s interpretation) all relevant questions of law.
- Increase professional staff levels within OIRA (at no net cost to taxpayers).
—Diane Katz is a Senior Research Fellow for Regulatory Policy in the Thomas A. Roe Institute for Economic Policy Studies, of the Institute for Economic Freedom, at The Heritage Foundation.
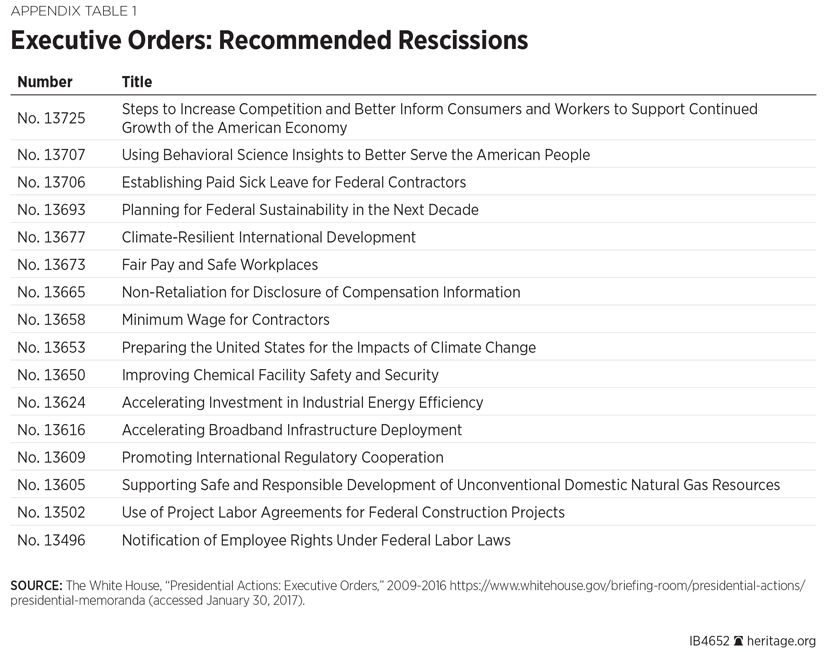
Appendix 1: Recommended Rescissions of Executive Orders, Guidance and Memoranda
Appendix 2
Appendix 3: Evolution of Regulatory Review
Regulatory review originated under President Richard Nixon in 1971. Then–OMB Director George Shultz directed the EPA’s then-Administrator William Ruckelshaus to submit proposed regulations to the OMB 30 days before publication, and to include analyses of the regulatory objectives, alternatives, and costs and benefits.
President Gerald Ford issued Executive Orders 11821 and 11949, directing regulatory agencies to prepare inflation and economic impact statements. President Jimmy Carter followed with Executive Order 12044 on “Improving Government Regulations.” Congress thereafter established OIRA in the 1980 Paperwork Reduction Act, which required agencies to obtain OMB approval to collect information from the public.
In 1982, President Ronald Reagan enhanced regulatory review with Executive Order 12291, which directed agencies to conduct regulatory impact analyses for all major rules, and declared: “Regulatory action shall not be undertaken unless the potential benefits to society for the regulation outweigh the potential costs to society.”
President Bill Clinton’s Executive Order 12866 instructed agencies to assess the costs and benefits of all regulatory alternatives, including not regulating. In deciding among the options, agencies were directed to select approaches that maximize net benefits (unless a statute requires otherwise). EO 12866 also stated explicitly that OIRA’s regulatory review is intended to make regulations “consistent with…the President’s priorities.”
President George W. Bush slightly amended Clinton’s order in EO 13258, which eliminated the role of the Vice President in regulatory review. (Shortly before EO 13258, Congress enacted the Information Quality Act, which directed the OMB to issue guidelines to federal agencies for “ensuring and maximizing the quality, utility, objectivity and integrity of information disseminated by Federal agencies,” including cost-benefit analyses.)
President Bush also issued Executive Order 13422, which required agencies to identify in writing the specific market failure or problem that the proposed regulation was intended to address, and to assess whether regulation was warranted.
For his part, President Barack Obama issued Executive Orders 13563 and 13610. The first reaffirmed the regulatory review principles in Clinton’s EO 12866, and directed agencies to consider “how best to promote retrospective analysis of rules that may be outmoded, ineffective, insufficient, or excessively burdensome.” Obama’s EO 13610 expanded on his directives for retrospective review by requiring agencies to “invite, on a regular basis…public suggestions about regulations in need of retrospective review and about appropriate modifications to such regulations.”